21 CFR Part 11 Module
eSign's 21 CFR Pt. 11 Module is a free module included with an Enterprise licence, and ensures that your organisation meets FDA regulations for the use of electronic signatures.
We're trusted by






E-Sign’s Compliance with 21 CFR Part 11
eSign provides a 21 CFR Part 11 module that ensures you comply with the US FDA’s 21 CFR Part 11 electronic signature regulations.
Our validated Part 11 module includes specific eSignature features for authentication, reason for signature, and signature manifestation. These tools ensure regulatory compliance while making the signing process faster, more cost-effective, and convenient for all parties involved.
Simplify compliance with eSign.
How is eSign Compliant with 21 CFR Pt. 11?
Our 21 CFR Pt. 11 module supports compliance through our leading electronic signature platform. The core elements of our 21 CFR Part 11 eSignature module include:
- Creation of a new 21 CFR Part 11 account
- Honour receipt signing order
- Controls for open systems
- Role-based access and multi-factor authentication
- Reason for signature (signing reason)
These features help to ensure that life science organisations are compliant with the regulation whilst using secure eSignatures, to streamline the process for completing important documents in a faster, more cost effective, and efficient way.
For more information, please download our Compliance Validator.

Generate accurate and complete copies of records.
Physical & Electronic Documents
Part of the requirements for compliance with 21 CFR Pt. 11 is that the system must have the ability to generate accurate and complete copies of records in both human readable and electronic form, suitable for review and inspection and copying by the FDA.
eSign provides the ability for authorised users to retrieve and export digitally signed documents along with a system generated digital audit history of signing events and an exportable Certificate of Completion. All documents are also digitally signed by eSign, which provides an open standards method to verify document integrity.

Control who can access the system and documents.
Restricted Access & User Permissions
21 CFR Part 11 states that the system must use authority checks to ensure that only authorised individuals can use the system, electronically sign a record, access the operation or computer system input or output device, alter a record, or perform the operation at hand.
eSign maintains a list of users, roles, access rights, and permissions within the system. The combination of email address and password identify a user, and the password is used to authenticate access to the system.
We also provides optional advanced authentication methods to validate the identity of all transacting parties, such as one time pass codes sent to mobile devices and knowledge-based authentication as well as PIN protection on envelopes.

Watch the video tutorial.
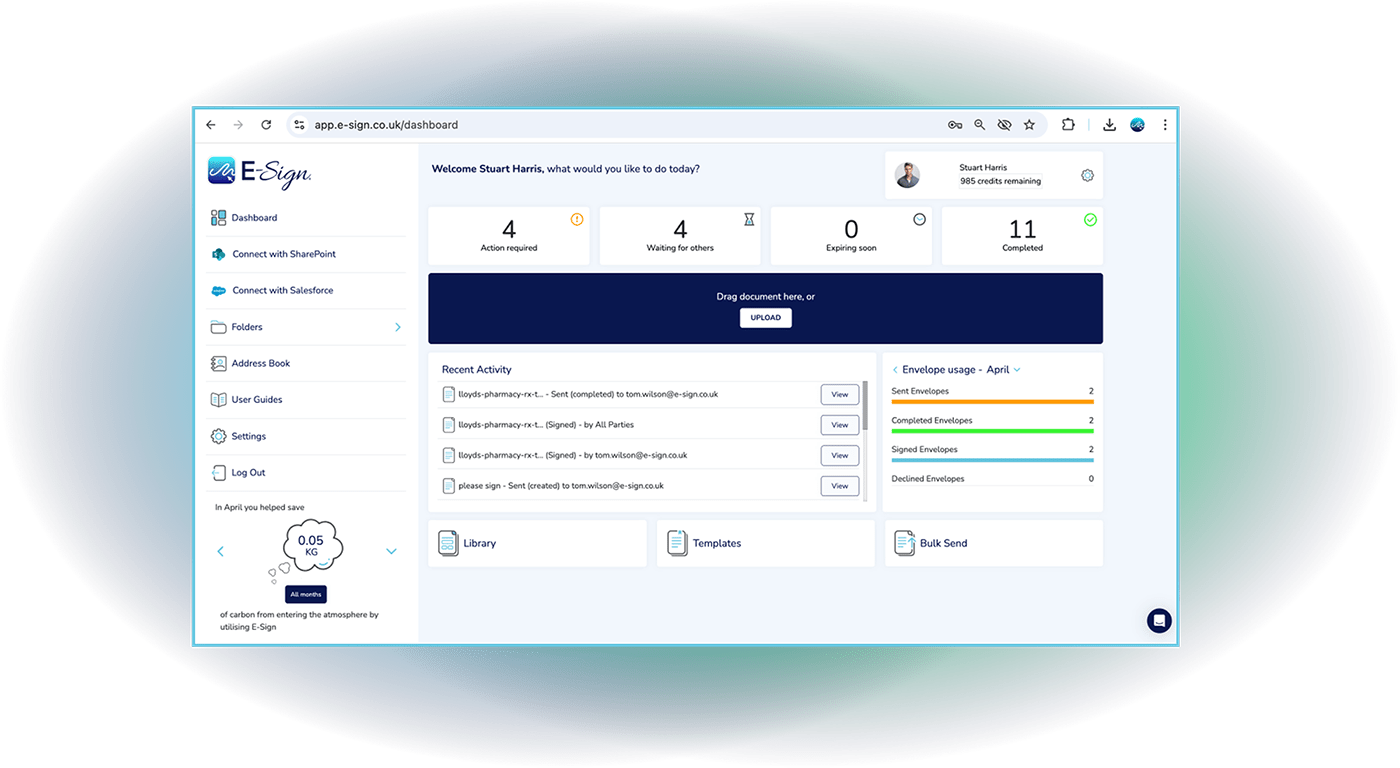
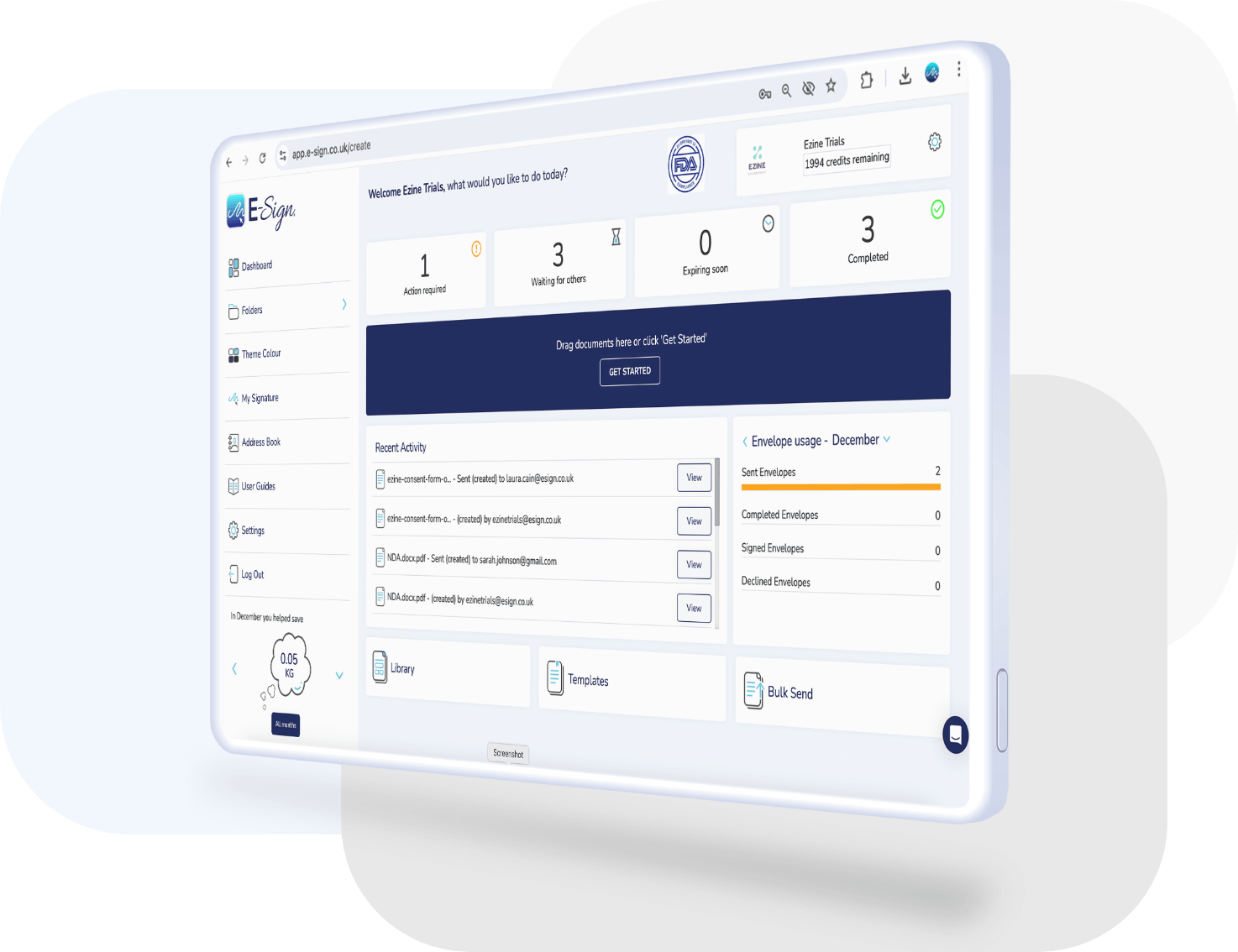
eSign 21 CFR Part 11 Module
Maintain compliance with FDA regulations by sending envelopes from an eSign 21 CFR Part 11 account. Recipients have the ability to state their reason for signing, as well as authenticating their identity before accessing the envelope.
Watch our video tutorial to see just how easy it is to send envelopes from an eSign CFR Part 11 account.
Integrate with Leading Tools
Create bespoke workflows to suit your business with a range of application integrations.















UK Government’s high-performance network
eSign is a UK government approved supplier
eSign is the only eSignature provider trusted on the UK Public Service Network (PSN) – proof that we meet the highest security, compliance, and interoperability standards.
We’re also on the Government’s Digital Marketplace, which enables buyers to select from a pre-approved list of suppliers.
For public sector organisations, this means your procurement and compliance teams get the reassurance of government-level due diligence already done – reducing risk and making roll-out smoother.
Benefits of eSign for 21 CFR Pt. 11 Compliance

Improve Operational Efficiency
Ensure you’re never held back by paper-based and manual processes with our digital signature and document platform.

Simplified Document Management
Once your documents have been digitally signed, you can easily store them securely within the eSign platform or download them to file in your chosen file structure.

Compliant Documents
eSign's platform is ISO 27001, ISO 9001 accredited and is 21 CFR Part 11, HIPAA, eIDAS and EMA compliant.
Supported by Full Audit Trail
Ensure compliance with a full audit trail and a transparent process, easily retrieving data at any time.

Faster Completion
Streamline your processes by sending all of your digital documents securely via eSign and allow users to sign documents in a matter of minutes.

Advanced Authentication
Validate the identity of all transacting parties, such as one time pass codes and knowledge-based authentication.
Featured Industries
Discover eSignature solutions and use cases in your industry.
eSign offers complete digital document solutions for healthcare organisations, streamlining processes, efficient transactions, and cost-effective services.
Learn More
Digital solutions for accountancy and tax, e-signatures for HMRC documents, book keeping and integrations into leading softwares.
Learn More
Streamline document turnaround and completion, increase compliance and elevate contract reviews with a digital document management solution.
Learn More
eSign offers digital solutions for educational organisations, from student enrolment, financial aid documents, HR documents and more.
Learn More
Digitise key financial agreements and workflows, do business faster and eliminate slow manual processes with an electronic signature.
Learn More
eSign provides digital solutions for organisations and individuals across all industries.
Learn More
Technical Checklist
- All signed documents are supported by a digital signature certificate.
- Ensure high standards of security and compliance with a full audit trail.
- Require recipient to be authenticated to access envelope.
- Allow account administrators to specify password criteria.
- A unique digital fingerprint is generated with each new signature.
- Download audit trail and all enclosed documents within the envelope
Frequently Asked Questions
How does eSign ensure compliance with 21 CFR Pt.11?
eSign has a 21 CFR Pt. 11 validation module that supports compliance through our leading electronic signature platform.
Our validated Part 11 module includes specific eSignature features for authentication, reason for signature, and signature manifestation.
These tools ensure regulatory compliance while making the signing process faster, more cost-effective, and convenient for all parties involved.
For more information, get in touch with our Digital Transformation team.
Where can I get the technical details of eSign's Part 11 support?
For the technical details of how our platform can assist your organisation with maintaining Part 11 compliance, download our 21 CFR Pt. 11 Technical Guide or get in touch with our Digital Transformation team.
What are the benefits of eSign for life sciences?
There are several benefits of using E-Sign for life science organisations, such as reducing cycle times for reviews and and approvals. Life science companies typically involve various complex processes which require collaboration with internal and external stakeholders. The use of traditional paper-based methods of using and exchanging documents has become outdated for these technological industries and can slow down product development.
With E-Sign, documents can be created, signed, and sent in just a few clicks, significantly reducing turnaround and improving communication for life science teams. This is also beneficial if your organisation has stakeholders based in different locations as E-Sign documents can be securely accessed from any device in any location.
Another important benefit of E-Sign for life sciences is that there is a reduced risk of human error. Life science is a precise industry that requires a high level of accuracy and through our impressive document management system features, you can implement automation in your workflow and mitigate mistakes as much as possible.
By maintaining compliance with 21 CFR part 11, E-Sign has become an industry leading provider for life science organisations. We can support companies with an effective digital platform that can meet and exceed their requirements both in terms of regulations and legality, and their internal processes in improving overall operational efficiency.
What are the 21 CFR Pt.11 requirements?
In order for the FDA to accept eSignatures in place of handwritten signatures, they must meet a specific set of criteria:
- The printed name of the signer
- A unique user ID
- The date and time of the signature
- Digital adopted signature
- The meaning of the signature (“signing reason”)
Other requirements also include:
- Each eSignature must be unique to only one individual and not reused by anyone else
- The identity of the signer must be verified before the electronic signature is applied
- There must be additional evidence from the e-signature provider that certify that the signature is legally binding
- If the signature is not based on biometrics, it must use two distinct components for identification such as an ID code and password
- The eSignature platform used must have safeguards in place to prevent unauthorised access to documents and stop unpermitted use of identification codes and passwords
Who is required to be compliant with 21 CFR Pt.11?
Any organisation that is regulated by the FDA or carries out activities that are related to FDA-regulated products are required to comply with 21 CFR part 11. These often include but are not limited to the below industries:
- Food and beverage manufacturers
- Cosmetic manufacturers
- Clinical laboratories
- Medical device manufacturers
- Pharmaceutical companies
- Contract research and manufacturing organisations
- Biotechnology companies
What happens if you don’t comply with CFR Pt.11?
If you’re required to comply with the regulation and you are not, you can face serious consequences such as:
- Warning letters
- Initiation of disqualification proceedings
- Notices of inquiry
- Criminal fines
- FDA-regulated products seized
- Recalls
- Criminal prosecution
- Retention orders