Electronic Signature Solutions for Life Sciences
Equip your life sciences organisation with a digital document platform, allowing for a joined up agreement process, improved efficiency and quicker document turnaround.

Trusted By
eSign Solutions for Life Sciences
eSign can help your life sciences organisation increase compliance, achieve digitalisation and simplify document management. Say goodbye to inefficient processes that slow down your progress.
With eSign’s 21 CFR Part 11 compliant electronic signature, you can save your organisation time on daily administration, increase compliance and free up time to spend on vital research and development. Collaborate with colleagues and customers more efficiently and enable you and your clients to sign and return paperwork digitally, in just a few clicks.
Make document security a priority.
Ensure Trust and Compliance with Advanced eSignatures
Life sciences is a highly regulated industry with many important documents being handled that require a secure signing process. E-Sign’s advanced electronic signatures are fully compliant with 21 CFR Part 11 and HIPPA, making us the ideal solution for your document workflows.
E-Sign is committed to maintaining the security and privacy of your documents and has robust protocols in place to ensure this. Each signature added through the platform comes with an advanced audit trail and has built-in identity verification through multi-factor authentication to protect the validity and integrity of your documents.

eSign securely and increase document turnaround.
Improve Document Turnaround and Speed to Market
With eSign’s functionalities guiding your document digitalisation, your life sciences organisation can benefit from streamlined processes and simplified document management. Paper-based processes are inefficient and out of place in a fast-paced clinical environment; they slow down your research and add to development costs.
Ensure documents are sent for signature securely and increase document turnaround. Enable your life sciences organisation to focus on product development, sales activity and avoid mistakes on sensitive documents.

Simplify compliance with eSign.
eSign's 21 CFR Part 11 Validation Module
If your life sciences organisation needs to comply with FDA regulation’s, you will be required to follow the 21 CFR Part 11 legislation on the use of electronic signatures. eSign has a 21 CFR Part 11 Validation Module available, that ensures you comply with the US FDA’s 21 CFR Part 11 electronic signature regulations.
eSign go above and beyond the legislative requirements set out by legal frameworks to ensure ultimate compliance and legality. Our eSignature platform is also compliant with HIPAA, eIDAS and EMA regulation for use in the life sciences sector.

Eliminating paper-based administration.
Save Time and Resources
By eliminating paper-based administration, your life sciences organisation not only stands to benefit from substantial time savings but also cost savings. By digitalising your document management, your business can eliminate its current spending on paper, printing supplies and postage fees, as well as the cost of filing folders, envelopes, and storage cabinets.
When time is money, optimising previously inefficient processes will also have a direct impact on your company’s processes, allowing you to maximise your profitability through greater efficiency and productivity. Hold-ups with paperwork can slow down vital research and add to development costs. Going digital allows you to improve efficiency and help keep your project within budget.

Electronic signature and document management platform.
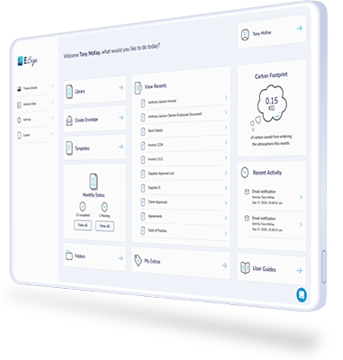
E-Sign Powering your Process
Ditching paper-based processes can help your organisation benefit from more efficient ways of working including:
- Peer Review Documents
- Patient Review Documents
- Patient Consent
- Clinical Trial Documents
- Audit and Compliance Paperwork
- 21 CFR Part 11 Compliance
With eSign’s electronic signature and document management platform, you can eliminate paper-based administration, moving document processes online to improve efficiency, reduce turnaround times and facilitate productive collaboration.

Integrate with Leading Tools
Create bespoke workflows to suit your business with a range of application integrations.














Benefits for Life Sciences

Improve Operational Efficiency
Ensure you’re never held back by paper-based and manual processes with our digital signature and document platform.

Simplified Document Management
Once your documents have been digitally signed, you can easily store them securely within the E-Sign platform or download them to file in your chosen file structure.

Compliant Documents
eSignature transactions are a legally binding method for almost all business document transactions and are 21 CFR Part 11, HIPAA, eIDAS and EMA compliant.
Supported by Full Audit Trail
Ensure compliance with a full audit trail and a transparent process, easily retrieving data at any time.

Faster Completion
Streamline your processes by sending all of your digital documents securely via E-Sign and allow users to sign documents in a matter of minutes.

User-Friendly Documents
With easy digital signing, you can make it simple to obtain patient consent or clinical trial information in just a few clicks.
Our Services
Create, eSign, send, and track documents all from within our unified system to streamline your workflows.
eSignatures
Complete documents faster with secure and legally binding electronic signatures that can be applied from any device.
Learn More
Web Forms
Allow customers to populate and sign documents on your website, app or via email. Collect the information you require in an interactive format.
Learn More
eWitnessing
Enable signers to identify witnesses without the need for them to be physically present with conveyancer-certified eWitnessing.
Learn More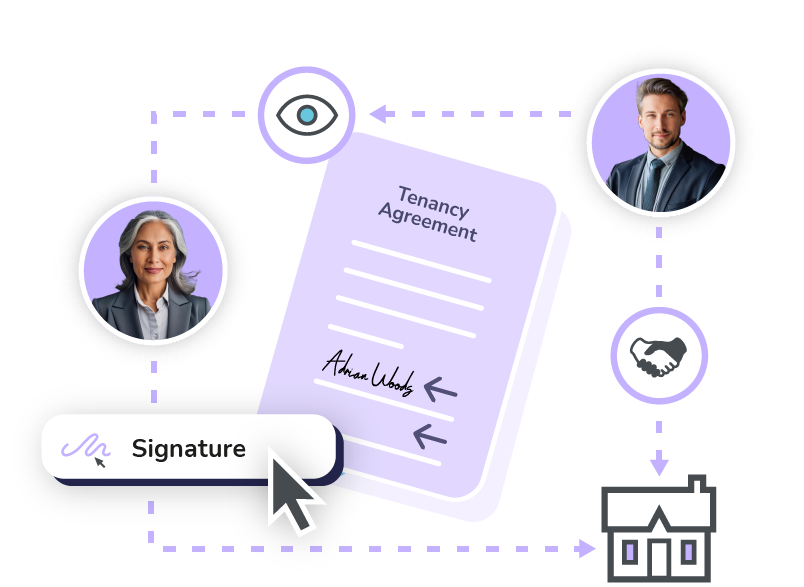
Personalised Email
Ensure strong, uniformed branding across all your communications and send documents for eSignature with personalised emails.
Learn More
eConsent
Enhance the patient experience, save consultant time and standardise information delivery processes with an eConsent solution.
Learn More
API/Developer Hub
Customise and extend the functionality of your apps to meet your unique business needs with eSign’s API.
Learn More

UK Government’s high-performance network
The Only Trusted Digital Signature Provider on the Public Service Network
eSign is the only electronic signature provider trusted on the Public Service Network. The PSN is the UK Government’s high-performance network, which enables public sector organisations to work together, reduce duplication and share resources.
Government organisations and health trusts access the PSN for secure and trusted digital services, that meet strict regulatory requirements and provide assurance that the service they access has the highest security standards, is exceptionally reliable, and can address issues within a rapid timeframe.
With the rapidly growing digitisation of document management and cloud-based solutions, the need for trustworthy providers has never been more important. The E-Sign document management and electronic signature solution provides businesses with improved efficiencies and major cost savings, creating enhanced advantages over competitors.
Frequently Asked Questions
Can eSign support 21 CFR Part 11 Compliance?
Our 21 CFR Pt. 11 Validation Module ensures that your organisation meets FDA regulations for the use of electronic signatures.
Our Part 11 module includes specific eSignature features for authentication, reason for signature, and signature manifestation.
These tools ensure regulatory compliance while making the signing process faster, more cost-effective, and convenient for all parties involved.
Who is required to be compliant with 21 CFR Part 11?
Any organisation that is regulated by the FDA or carries out activities that are related to FDA-regulated products are required to comply with 21 CFR part 11. These often include but are not limited to the below industries:
- Food and beverage manufacturers
- Cosmetic manufacturers
- Clinical laboratories
- Medical device manufacturers
- Pharmaceutical companies
- Contract research and manufacturing organisations
- Biotechnology companies